225Ac-FL-020 is a novel PSMA-targeting radionuclide drug conjugate (RDC) with superior in vivo anti-tumor activity
Authors
F. Liu1, J. Zhang1, J. Yang1, K. T. Thrane2, M. W. Hallund2, R. V. Grønlund2, N. C. L. Wong1;
1Full-Life Technologies Limited, Shanghai, China, 2Minerva Imaging ApS, Ølstykke, Denmark
Disclosures
F. Liu,
Full-Life Technologies Limited Employment.
J. Zhang,
Full-Life Technologies Limited Employment.
J. Yang,
Full-Life Technologies Limited Employment.
K. T. Thrane, None..
M. W. Hallund, None..
R. V. Grønlund, None.
N. C. L. Wong, Full-Life Technologies Limited Employment
Abstract
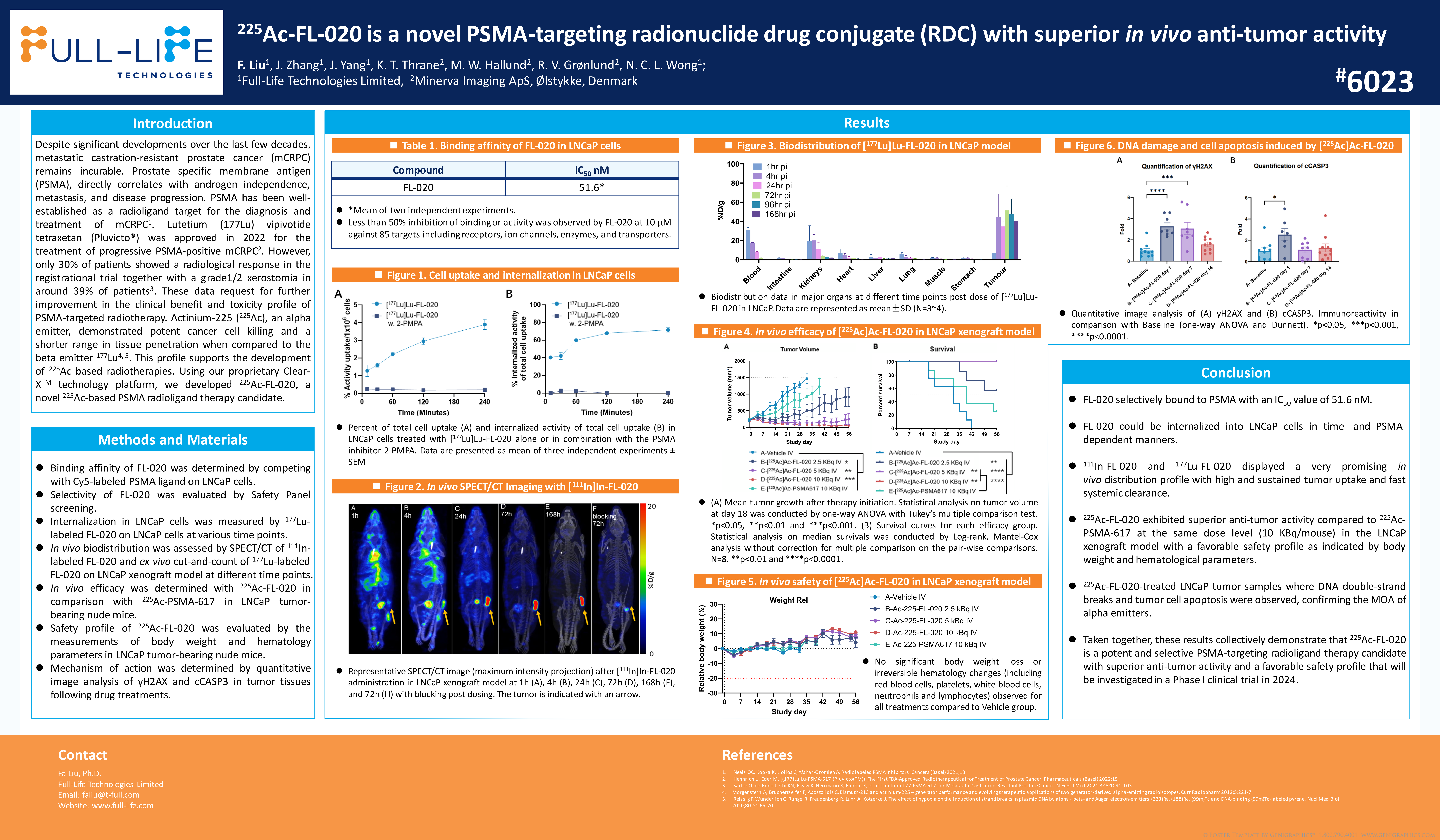
Despite significant developments over the last few decades, metastatic castration-resistant prostate cancer (mCRPC) remains incurable. Prostate specific membrane antigen (PSMA), a prostate cancer-specific membrane marker which directly correlates with androgen independence, metastasis, and disease progression, has been well-established as a radioligand target for the diagnosis and treatment of mCRPC. Lutetium-177 (177Lu)-PSMA-617 (PLUVICTOTM) was approved in 2022 for the treatment of progressive PSMA-positive mCRPC. However, only 30% of patients showed a radiological response in the registrational trial. Meanwhile, recent clinical experience has shown very limited efficacy in low PSMA expressing patients. These data collectively request for further improvement in the clinical benefit of PSMA-targeted radiotherapy. Actinium-225 (225Ac), an alpha emitter, is significantly more potent on cancer cell killing and has a shorter range in tissue penetration when compared to the beta emitter 177Lu. Such a profile supports the development of 225Ac based radiotherapies. Using our proprietary Clear-XTM technology platform, we developed 225Ac-FL-020, a novel 225Ac-based PSMA radioligand therapy candidate which has demonstrated prominent pre-clinical properties. We have assessed the binding affinity against PSMA of the non-labeled vector FL-020 in vitro. The in vivo biodistribution profile of FL-020 was characterized by SPECT/CT imaging and biodistribution using Indium-111 (111In)-FL-020 in PSMA high LNCaP tumor-bearing nude mice. In addition, anti-tumor activities of 225Ac-FL-020 were evaluated in the LNCaP xenograft model and directly compared to 225Ac-PSMA-617. FL-020 was found bound to LNCaP cells with an IC50 value of 51.55 nM. Meanwhile, the off-target screening showed that less than 50% inhibition of binding or activity was observed by FL-020 at 10 µM against 85 targets including receptors, ion channels, enzymes, and transporters, indicating the high selectivity of FL-020. Moreover, 111In-FL-020 displayed a very promising in vivo distribution profile with high and sustained tumor uptake and fast systemic clearance. Furthermore, 225Ac-FL-020 exhibited superior anti-tumor activity compared to 225Ac-PSMA-617 at the same dose level (10 KBq/mouse) in the LNCaP xenograft model with a favorable safety profile as indicated by body weight and hematological parameters. A mechanistic study was also conducted in 225Ac-FL-020-treated LNCaP tumor samples where DNA double-strand breaks and tumor cell apoptosis were observed, confirming the MOA of alpha emitters. Taken together, these results collectively demonstrate that 225Ac-FL-020 is a potent and selective PSMA-targeting radioligand therapy candidate with superior anti-tumor activity and a favorable safety profile warranting further clinical development.