Full-Life Technologies to Present Three Top Rated Oral Presentations at the 37th Annual Congress of the European Association of Nuclear Medicine
Shanghai, China, and Heidelberg, Germany – October 16, 2024 – Full-Life Technologies ("Full-Life", the "Company"), a fully integrated global radiotherapeutics company, today announced that the Company will be presenting at the 37th Annual Congress of the European Association of Nuclear Medicine ("EANM"), which will be held in Hamburg, Germany, from October 19-23, 2024. Juan Zhang, Ph.D, Senior Vice President and Head of Biology, and Taishan Hu, Ph.D, Senior Vice President and Head of Medicinal Chemistry, will give three preclinical presentations which have been accepted as Top Rated Oral Presentation ("TROP").
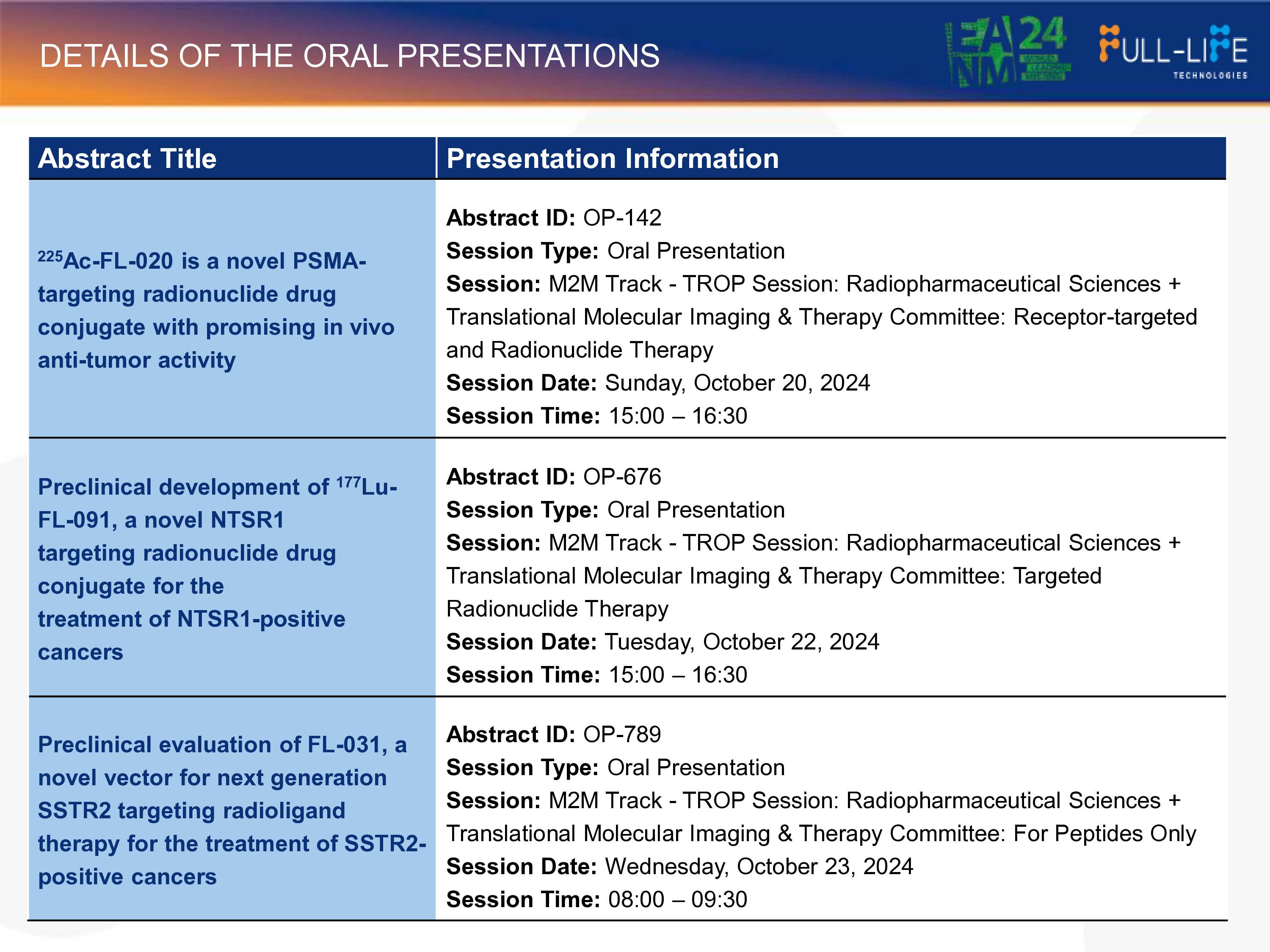
Details of these presentations are given in the table below:
The abstract book is available at https://link.springer.com/article/10.1007/s00259-024-06838-z.
About 225Ac-FL-020
225Ac-FL-020 is a novel, potential best-in-class, next-generation PSMA-targeting radionuclide drug conjugate ("RDC") that entered global Ph1 clinical studies in 2024. Its targeting vector, FL-020, was discovered using Full-Life's proprietary UniRDC™ platform, which enables significant improvement of drug uptake in the tumor while maintaining fast systemic clearance. In pre-clinical models, 225Ac-FL-020 has demonstrated potent anti-tumor activity and a favorable safety profile. The U.S. FDA has granted 225Ac-FL-020 Fast Track Designat ion.
ion.
About FL-091
FL-091 is a novel small-molecule radionuclide drug conjugate ("RDC") vector targeting Neurotensin receptor 1 ("NTSR1") positive solid tumors. Overexpression of NTSR1 has been associated with disease progression in multiple types of cancers, including colorectal, breast, pancreatic, and head and neck cancers, etc. FL-091 RDCs have demonstrated favorable biodistribution profiles and enhanced binding affinity to NTSR1, as well as encouraging anti-tumor activities in preclinical studies. In July 2024, Full-Life licensed exclusive worldwide rights for the clinical research, development, manufacturing and commercialization of FL-091 to SK Biopharmaceuticals.
About FL-031
FL-031 is a novel peptide-based vector targeting SSTR2 positive solid tumors. In preclinical studies, 177Lu-FL-031 has shown promising biodistribution profiles and anti-tumor activity in both high and low SSTR2 expression cell-line-derived xenograft ("CDX") models. With the Ac-225-labeled FL-031 in preclinical development as a potential targeted alpha-therapy, Full-Life aims to expand the indication of SSTR2-targeted RDC therapies beyond gastroenteropancreatic neuroendocrine tumors ("GEP-NETs") to others including small cell lung cancer ("SCLC").
About Full-Life Technologies
Full-Life Technologies ("Full-Life") is a fully integrated global radiotherapeutics company with operations in Belgium, Germany, and China. We aim to own the entire value chain for radiopharmaceutical research & development, production & commercialization to deliver clinical impact for patients. The Company endeavors to tackle fundamental challenges affecting radiopharmaceuticals today by pioneering innovative research that will shape the treatments of tomorrow. We are comprised of a team of fast-moving entrepreneurs and seasoned scientists with a proven history of success in the life sciences, alongside radioisotope research and clinical development.